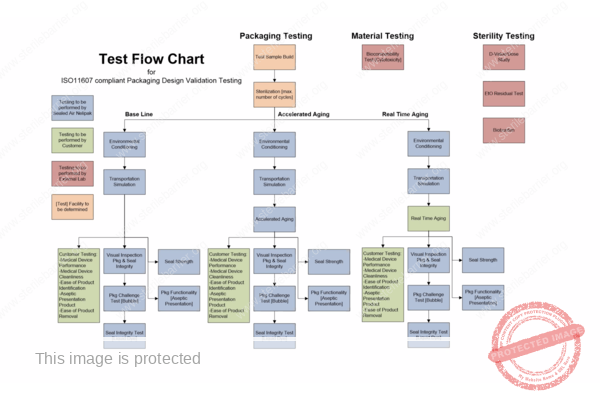
The key functionality of packaging for terminally sterilised medical devices is to allow for sterilisation and to maintain sterility until the point of use in a healthcare setting. Package designs must be validated (qualified), and the packaging process must be validated and controlled. Validation requires the use of test methods, which must also be properly validated as well following the requirements of EN ISO 11607.
Design and process validation are often combined in an effort to mitigate the validation costs. When doing so, it is important to structure testing properly so that any failures can be investigated and any retesting is minimised.